AOD-9604
AOD-9604 is a synthetic 16-amino acid peptide fragment derived from the C-terminal domain of human growth hormone (amino acids 176-191) with a stabilizing tyrosine substitution at the N-terminus. Originally developed by Metabolic Pharmaceuticals Ltd. in Australia during the 1990s as an "Anti-Obesity Drug," it was designed to harness growth hormone's lipolytic properties while avoiding broader metabolic and growth-promoting effects.
Despite completing six human clinical trials involving over 900 participants, AOD-9604 failed to achieve statistical significance in its largest Phase IIb trial and development was terminated in 2007. The peptide lacks regulatory approval from any major health authority worldwide. Its selective mechanism targeting adipose tissue without affecting IGF-1 pathways or glucose homeostasis distinguishes it from full-length growth hormone therapy, offering unique research applications in metabolic studies and potential cartilage repair applications.

Overview
AOD-9604 exhibits rapid degradation in biological systems through amino-terminal truncation, with sequential amino acid removal being the primary metabolic pathway. The peptide maintains structural similarities to the homologous region of naturally occurring human growth hormone while demonstrating improved stability through its synthetic modifications. Detection in plasma and urine is possible using specialized LC/MS/MS assay methods for up to several days post-administration.

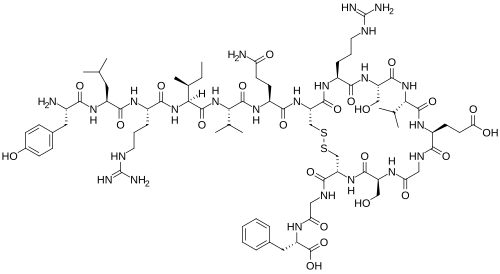
Chemical structure & Properties
- Molecular Formula: C₇₈H₁₂₃N₂₃O₂₃S₂
- Molecular Weight: 1815.1 Daltons
- Sequence: Tyr-Leu-Arg-Ile-Val-Gln-Cys-Arg-Ser-Val-Glu-Gly-Ser-Cys-Gly-Phe
- Disulfide Bridge: Cys7-Cys14 (cyclic structure)
- CAS Number: 221231-10-3
- Half-life: Approximately 4 minutes in plasma (rapid degradation)
- Stability: Enhanced compared to native hGH C-terminus through N-terminal tyrosine modification
Mechanism of Action
AOD-9604 exerts its biological effects through multiple interconnected molecular mechanisms:
Clinical Applications and
Research Evidence

Current Clinical Evidence
Safety Profile and Considerations
Regulatory Status and
Legal Considerations
Global Regulatory Status
No Approved Medical Use:
- FDA Status: Not approved for any therapeutic indication
- International Status: No regulatory approval from any major health authority worldwide
- Development Status: Classified as investigational compound under pre-clinical/clinical development
WADA Anti-Doping Status
Prohibited Substance Classification:
- Category: S0 - Non-Approved Substances (prohibited at all times)
- Rationale: No current approval for human therapeutic use
- Athletic Use: Banned in all competitive sports
- Detection: Urine testing available for anti-doping purposes
Legal Availability
Research Chemical Status:
- Commercial Availability: Available through research chemical suppliers
- Quality Control: Variable standards depending on supplier
- Regulatory Oversight: Subject to research chemical regulations
- Medical Use: Not approved for clinical therapeutic applications
Regional Variations:
- United States: Research use only, not FDA-approved
- Australia: Schedule 4 prescription-only medicine classification
- European Union: No medical approval, research use restrictions apply
Administration and Dosing
Considerations
Reconstitution and Storage:
- Formulation: Lyophilized powder requiring reconstitution
- Storage Conditions: 2°C to 8°C before reconstitution
- Stability: Use within specified timeframe after reconstitution
- Injection Sites: Abdominal subcutaneous tissue with rotation
Clinical Considerations
Important Limitations:
- No Established Medical Dosing: Absence of approved therapeutic protocols
- Research Use Only: Not intended for clinical therapeutic applications
- Individual Variability: Response varies significantly between individuals
- Medical Supervision: Professional oversight required for any investigational use

Mechanistic Studies:
- Elucidation of non-beta-3-AR mediated lipolytic pathways
- Investigation of cartilage repair mechanisms
- Cellular and molecular target identification
- Tissue-specific metabolic effects characterization
Clinical Research Needs:
- Large-scale efficacy trials in diverse populations
- Long-term safety assessment and monitoring
- Optimal dosing strategies for various applications
- Combination therapy investigations with other peptides
Therapeutic Applications:
- Cartilage repair and osteoarthritis treatment validation
- Metabolic syndrome and NAFLD research
- Age-related metabolic dysfunction studies
- Selective adipose tissue research applications
Emerging Research Areas
Novel Applications Under Investigation:
- Joint and Cartilage Health: Potential osteoarthritis treatment and cartilage regeneration
- Metabolic Research: Tool for studying selective fat metabolism pathways
- Combination Therapies: Synergistic effects with other peptides (e.g., BPC-157)
- Tissue Engineering: Applications in regenerative medicine research


Conclusion
AOD-9604 represents a unique synthetic peptide with well-characterized safety profile but limited proven efficacy for its originally intended obesity indication. The extensive clinical safety database from over 900 participants across six controlled trials establishes AOD-9604 as well-tolerated with minimal adverse effects. However, failure to demonstrate consistent efficacy in large-scale trials led to development termination in 2007.
Its selective mechanism targeting adipose tissue metabolism without affecting IGF-1 pathways or glucose homeostasis makes it valuable for research applications studying fat metabolism and emerging cartilage repair investigations. The peptide's regulatory status as an unapproved investigational compound limits clinical applications while maintaining research utility.
Until comprehensive efficacy data becomes available, AOD-9604 remains primarily a research tool rather than a validated therapeutic intervention. Healthcare providers and researchers must carefully evaluate risk-benefit profiles within appropriate research frameworks and ensure regulatory compliance when considering AOD-9604 applications.
AOD-9604 SCIENTIFIC
DATA SUMMARY
Disclaimer: This information is provided for educational purposes only and does not constitute medical advice. AOD-9604 is not approved by the FDA or any major regulatory authority for therapeutic use. The peptide is classified as a research chemical and prohibited for use in competitive athletics by WADA.
Patients and researchers should consult with qualified healthcare providers and ensure compliance with applicable regulations before considering any use of AOD-9604.
