ARA-290
ARA-290 is a synthetic 11-amino acid peptide derived from the tissue-protective domain of erythropoietin (EPO), specifically engineered to retain neuroprotective and tissue-protective properties while eliminating erythropoietic activity. This innovative therapeutic agent activates the innate repair receptor (IRR), also known as the common β-receptor (CD131), which plays a crucial role in tissue protection, repair, and regeneration processes.
Unlike native erythropoietin, ARA-290 does not stimulate red blood cell production, making it suitable for therapeutic applications without the cardiovascular risks associated with increased hematocrit. The peptide has gained significant attention in regenerative medicine and neurology due to its demonstrated efficacy in treating diabetic neuropathy, chronic neuropathic pain, and inflammatory conditions through its unique mechanism of promoting neural repair and reducing inflammation.

Overview
ARA-290 was specifically designed through peptide engineering to isolate the tissue-protective properties of erythropoietin from its hematopoietic effects. The peptide maintains the critical binding domain necessary for IRR activation while eliminating sequences responsible for erythropoiesis. The peptide demonstrates high bioavailability (>85%) via subcutaneous injection and is metabolized primarily in the liver with renal elimination.

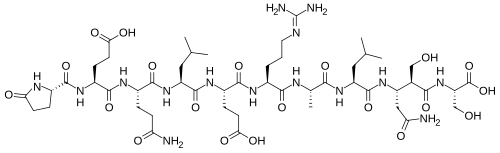
Chemical structure & Properties
- Molecular Formula: C59H95N17O22
- Molecular Weight: 1,382 Da
- Sequence: 11 amino acids derived from erythropoietin's tissue-protective domain
- Half-life: Approximately 6-8 hours following subcutaneous administration
- Stability: Stable in lyophilized form, requires refrigeration when reconstituted
Mechanism of Action
ARA-290 exerts its therapeutic effects through multiple interconnected molecular pathways:
Clinical Applications and
Research Evidence

Current Clinical Evidence
Safety Profile and Considerations
Regulatory Status and
Legal Considerations
FDA Status
- Classification: Investigational drug under clinical development
- Approval Status: Not approved for commercial therapeutic use
- Research Status: Available for clinical trials and expanded access programs
- Regulatory Position: Phase II completed, Phase III studies needed for approval
International Status
- EMA: Orphan drug designation potential for rare neuropathic conditions
- Other Regions: Early-stage regulatory interactions initiated
- Clinical Access: Limited to research protocols and compassionate use cases
Legal Availability
- Commercial Status: Not legally available as prescription medication
- Market Presence: Limited to clinical trial participants
- Quality Control: Clinical-grade material produced under GMP conditions
- Clinical Use: Restricted to research settings and experimental protocols
Administration and Dosing
Considerations
The Paragon Method: Step-by-Step
Clinical Considerations
Important Notes:
- No FDA-approved dosing guidelines exist for human use
- Individual response may vary significantly
- Medical supervision required for any therapeutic application
- Quality and purity limited to clinical-grade preparations

Priority Research Areas
- Large-scale Phase III clinical trials for regulatory approval
- Long-term safety studies with extended follow-up periods
- Comparative effectiveness research against standard therapies
- Optimal dosing regimens for different neuropathic conditions
- Biomarker development for treatment response prediction
Emerging Applications
Research is investigating potential applications in:
- Central nervous system neuroprotection
- Stroke recovery and traumatic brain injury
- Age-related neurodegenerative diseases
- Inflammatory bowel disease and tissue protection
- Athletic performance and cognitive optimization


Conclusion
ARA-290 represents a promising therapeutic peptide with demonstrated benefits in clinical studies of diabetic neuropathy and chronic neuropathic pain. Its unique mechanism of action through innate repair receptor activation offers distinct advantages in neurological applications without the cardiovascular risks associated with traditional erythropoietin therapy.
The current evidence base includes positive Phase II clinical trial results with excellent safety profiles. However, the investigational status and need for Phase III studies necessitate careful consideration and medical supervision for any therapeutic application. Patients interested in ARA-290 therapy should engage in thorough discussions with qualified healthcare providers and explore participation in clinical trials or expanded access programs.
Future research will be critical in establishing the full therapeutic potential of ARA-290 across various neurological and inflammatory conditions. The peptide's novel approach to activating endogenous repair mechanisms positions it as a potentially transformative therapeutic tool in neurology and regenerative medicine.
ARA-290 SCIENTIFIC
DATA SUMMARY
Disclaimer: This information is provided for educational purposes only and does not constitute medical advice. ARA-290 is currently investigational and not approved by the FDA for routine therapeutic use. Access is limited to clinical trials and expanded access programs. Patients should consult with qualified healthcare providers before considering any experimental peptide therapy.
The content reflects current scientific literature and regulatory status as of 2025.
