GHK-Cu
GHK-Cu (Glycyl-L-Histidyl-L-Lysine-Copper) is a naturally occurring tripeptide-copper complex originally discovered in human plasma, serum, and urine. This bioactive compound represents one of the most extensively studied and clinically validated peptides in regenerative medicine, demonstrating remarkable therapeutic properties through its multifaceted mechanisms of action including collagen synthesis stimulation, anti-inflammatory effects, antioxidant activity, and tissue remodeling enhancement.
This peptide has gained considerable attention in dermatology and aesthetic medicine due to its demonstrated efficacy in wound healing, skin regeneration, hair follicle stimulation, and anti-aging applications. GHK-Cu exhibits unique stability as a copper chelate complex and maintains bioactivity across various physiological conditions, making it distinctive among therapeutic peptides with decades of clinical use and research validation.

Overview
GHK-Cu exists as a stable coordination complex where the copper ion is chelated by the histidine and terminal amino group, creating a distinctive blue-colored solution. The peptide demonstrates superior stability and biological activity compared to either component alone, and is metabolized through gradual peptide degradation with copper redistribution to tissue stores, with detectability up to 12 hours post-administration using standard analytical methods.

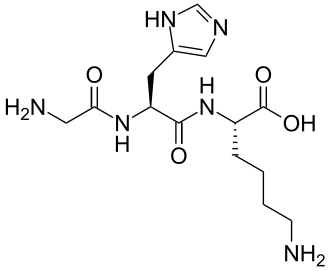
Chemical structure & Properties
- Molecular Formula: C14H24CuN6O4
- Molecular Weight: 404.93 Da (with copper), 342.43 Da (without copper)
- Sequence: Glycyl-L-Histidyl-L-Lysine complexed with Cu²⁺
- Half-life: 2-4 hours (plasma circulation)
- Stability: Stable copper chelate complex with enhanced bioavailability
Mechanism of Action
GHK-Cu exerts its therapeutic effects through multiple interconnected molecular pathways:
Clinical Applications and
Research Evidence

Current Clinical Evidence
Safety Profile and Considerations
Regulatory Status and
Legal Considerations
FDA Status
- Classification: Varies by administration route and concentration
- Topical Applications: Available in cosmetic and cosmeceutical formulations
- Injectable Forms: Currently prohibited for commercial compounding (2023)
- Regulatory Position: Research use available for investigational applications
WADA Status
- Classification: Not specifically prohibited in current regulations
- Athletic Use: Generally permitted in topical applications
- Testing: Limited detection methods for performance monitoring
Legal Availability
- Commercial Status: Widely available in topical formulations
- Market Presence: Concentrations from 0.1% to 2% in cosmetic products
- Quality Control: Wide variation in product quality and concentration accuracy
- Clinical Use: Professional supervision recommended for therapeutic applications
Administration and Dosing
Considerations
The Paragon Method: Step-by-Step
Clinical Considerations
Important Notes:
- Variable regulatory status for different delivery methods
- Individual response may vary significantly based on application method
- Professional supervision recommended for injectable therapeutic applications
- Product quality and concentration accuracy varies significantly between manufacturers

Priority Research Areas
- Large-scale randomized controlled trials for definitive efficacy establishment
- Standardized dosing protocols for various clinical applications
- Long-term safety studies with extended follow-up periods
- Comparative effectiveness research against established treatments
- Advanced understanding of gene expression mechanisms
Emerging Applications
Research is investigating potential applications in:
- Neurological applications and cognitive enhancement
- Systemic regenerative medicine applications
- Advanced tissue engineering and organ repair
- Personalized medicine approaches based on genetic factors
- Enhanced delivery systems and targeted therapy


Conclusion
GHK-Cu represents a well-established therapeutic peptide with demonstrated benefits in clinical studies of skin rejuvenation, wound healing, and tissue regeneration. Its unique mechanism of action through gene expression modulation and copper-mediated enzymatic enhancement offers distinct advantages in dermatological and aesthetic applications. The extensive research base includes decades of clinical use with excellent safety profiles.
The current evidence base demonstrates significant therapeutic potential with established safety across multiple administration routes. However, variable regulatory status and the need for larger clinical trials necessitate careful consideration and professional supervision for therapeutic applications. Patients interested in GHK-Cu therapy should engage in thorough discussions with qualified healthcare providers to explore appropriate formulations and administration methods.
Future research will continue to expand understanding of GHK-Cu's therapeutic applications across regenerative medicine. The combination of established clinical benefits, ongoing research developments, and excellent safety profile ensures continued importance in aesthetic medicine, dermatology, and tissue repair applications.
GHK-Cu SCIENTIFIC
DATA SUMMARY
Disclaimer: This information is provided for educational purposes only and does not constitute medical advice. GHK-Cu availability and regulatory status vary by jurisdiction and administration route. Injectable forms are currently prohibited for commercial compounding by FDA regulations as of 2023. Patients should consult with qualified healthcare providers before considering any peptide therapy.
The content reflects current scientific literature and regulatory status as of 2025.
