Ipamorelin
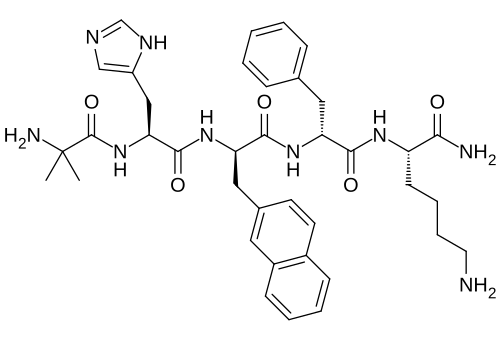
Ipamorelin is a synthetic pentapeptide consisting of 5 amino acids (Aib-His-D-2-Nal-D-Phe-Lys-NH2) classified as a growth hormone secretagogue receptor (GHSR) agonist, specifically targeting the ghrelin receptor. Originally developed by Novo Nordisk in the late 1990s, ipamorelin represents one of the most selective growth hormone-releasing peptides (GHRPs) available, demonstrating minimal activation of secondary hormone pathways compared to other compounds in its class.
This peptide has gained attention in anti-aging and regenerative medicine due to its ability to stimulate endogenous growth hormone (GH) and insulin-like growth factor-1 (IGF-1) release while maintaining a favorable safety profile. Ipamorelin's selectivity for GH release, without significantly affecting cortisol, prolactin, or acetylcholine pathways, distinguishes it from other growth hormone secretagogues and enhances its therapeutic potential.

Overview
Ipamorelin features several unique structural modifications, including the presence of 2-naphthylalanine (2-Nal) and D-amino acids, which contribute to its stability and receptor selectivity. The peptide is metabolized primarily through peptidase enzymes and eliminated through renal excretion, with metabolites detectable for up to 24 hours post-administration.
Chemical structure & Properties
- Molecular Formula: C38H49N9O5
- Molecular Weight: 711.85 Da
- Sequence: Aib-His-D-2-Nal-D-Phe-Lys-NH2
- Half-life: Approximately 2 hours (plasma elimination)
- Stability: Stable in aqueous solution when refrigerated, susceptible to degradation at room temperature
Mechanism of Action
Ipamorelin exerts its therapeutic effects through multiple interconnected molecular pathways:
Clinical Applications and
Research Evidence
.png)
Current Clinical Evidence
Safety Profile and Considerations
Regulatory Status and
Legal Considerations
FDA Status
- Classification: Investigational new drug (IND) - not approved for therapeutic use
- Approval Status: No FDA approval for human clinical applications
- Research Use: Limited to approved clinical trials and research protocols
- Regulatory Position: Requires further clinical development for therapeutic approval
WADA Status
- Classification: Prohibited under S2: Peptide Hormones, Growth Factors
- Athletic Use: Banned in competitive sports
- Testing: Detectable through specialized anti-doping analysis
- Violation Consequences: Subject to sporting sanctions and disqualification
Legal Availability
- Commercial Status: Not legally available as prescription medication
- Market Presence: Available through research chemical suppliers
- Quality Control: No regulatory oversight ensuring purity or potency
- Clinical Use: Restricted to research institutions and approved clinical trials
Administration and Dosing
Considerations
The Paragon Method: Step-by-Step
Clinical Considerations
Important Guidelines:
- No FDA-approved dosing recommendations exist
- Individual response varies significantly based on age, health status, and baseline GH levels
- Medical supervision strongly recommended for any therapeutic application
- Regular monitoring of IGF-1 levels and metabolic parameters advised
- Quality and authenticity of research chemicals cannot be guaranteed

Priority Research Areas
Clinical Development Priorities:
- Large-scale, long-term randomized controlled trials for safety and efficacy
- Standardized dosing protocols for specific therapeutic indications
- Comprehensive safety evaluation including carcinogenicity studies
- Drug interaction studies with commonly prescribed medications
- Optimal treatment duration and cycling protocols
Emerging Applications
Investigational Uses:
- Post-surgical recovery and wound healing enhancement
- Age-related cognitive decline and neuroprotection
- Metabolic syndrome and type 2 diabetes management
- Osteoporosis prevention and treatment
- Chronic fatigue syndrome and fibromyalgia
- HIV-associated wasting and muscle atrophy
Combination Therapies
Synergistic Research:
- Combination with CJC-1295 for prolonged GH elevation
- Integration with exercise and nutritional interventions
- Potential combination with other regenerative peptides
- Synergistic effects with hormone replacement therapies


Conclusion
Ipamorelin represents a promising and selective growth hormone secretagogue with demonstrated potential in anti-aging, body composition improvement, and metabolic enhancement. Its favorable selectivity profile, minimal side effects, and physiological hormone stimulation distinguish it from other peptides in its class.
However, the current clinical evidence base remains limited, with most data derived from small-scale, short-term studies. The lack of FDA approval, regulatory restrictions, and limited long-term safety data necessitate careful consideration and medical supervision for any therapeutic application.
Patients considering ipamorelin therapy should engage in thorough discussions with qualified healthcare providers to weigh potential benefits against risks and explore evidence-based treatment alternatives. Future research will be critical in determining ipamorelin's role in clinical medicine, particularly in healthy aging, metabolic disorders, and regenerative medicine applications. Until comprehensive clinical trials are completed, its use should remain limited to research settings under appropriate medical oversight.
IPAMORELIN SCIENTIFIC
DATA SUMMARY
Disclaimer: This information is provided for educational purposes only and does not constitute medical advice. Ipamorelin is not approved by the FDA for human therapeutic use. Patients should consult with qualified healthcare providers before considering any peptide therapy.
The content reflects current scientific literature and regulatory status as of 2025.
