PE-22-28
PE-22-28, also known as Mini-Spadin, is a synthetic heptapeptide consisting of 7 amino acids developed as an optimized analog of the naturally occurring peptide Spadin. Originally derived from research into TWIK-related K+ channel 1 (TREK-1) modulation, PE-22-28 represents a novel therapeutic approach to mood disorders and cognitive enhancement through selective potassium channel inhibition.
This peptide has gained considerable attention in neuropsychiatric medicine due to its demonstrated efficacy in preclinical models of depression, anxiety, and cognitive dysfunction. PE-22-28 exhibits unique pharmacological properties through TREK-1 channel blockade, offering rapid-onset therapeutic effects with improved tolerability compared to traditional antidepressants, making it distinctive among neurotherapeutic peptides.

Overview
PE-22-28 demonstrates superior pharmacokinetic properties compared to its parent compound Spadin, with structural modifications that enhance stability and extend duration of action. The peptide exhibits water solubility and maintains bioactivity across various physiological conditions, though it requires parenteral or intranasal administration due to poor oral bioavailability. The compound is metabolized through peptidase enzymes with sustained activity extending beyond 24 hours post-administration.

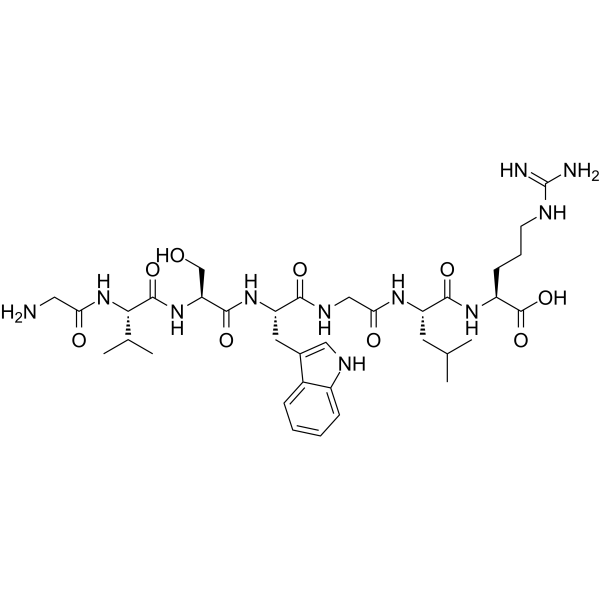
Chemical structure & Properties
- Molecular Formula: C35H54N12O9S
- Molecular Weight: 802.94 Da
- Sequence: 7-amino acid peptide (optimized Spadin analog)
- Half-life: >24 hours activity duration
- Stability: Enhanced in vivo stability through structural optimization
Mechanism of Action
PE-22-28 exerts its therapeutic effects through multiple interconnected molecular pathways:
Clinical Applications and
Research Evidence
.png)
Current Clinical Evidence
Safety Profile and Considerations
Regulatory Status and
Legal Considerations
FDA Status
- Classification: Investigational peptide - not approved for therapeutic use
- Approval Status: No FDA approval for human clinical applications
- Research Use: Limited to experimental protocols and research settings
- Regulatory Position: Requires comprehensive clinical development for therapeutic approval
WADA Status
- Classification: Potential concern under S0: Non-Approved Substances
- Athletic Use: Status unclear; consultation recommended for competitive athletes
- Testing: Detection methods may be limited due to novel compound status
Legal Availability
- Commercial Status: Not legally available as prescription medication
- Market Presence: Available through specialized research chemical suppliers
- Quality Control: No regulatory oversight ensuring purity or potency standards
- Clinical Use: Restricted to research institutions and experimental applications
Administration and Dosing
Considerations
The Paragon Method: Step-by-Step
Administration Routes
Primary Methods:
- Subcutaneous Injection: Most common route with optimal bioavailability
- Intranasal Administration: Alternative route for cognitive enhancement applications
- Intramuscular Injection: Suitable alternative with sustained absorption
- Oral Administration: Not recommended due to poor bioavailability
Clinical Considerations
Important Guidelines:
- No FDA-approved dosing recommendations exist for human use
- Individual response varies significantly based on neurochemical baseline
- Medical supervision strongly recommended for any therapeutic application
- Regular monitoring of mood and cognitive function advised during treatment
- Quality and authenticity of research compounds cannot be guaranteed

Priority Research Areas
Clinical Development Priorities:
- Large-scale human clinical trials for safety and efficacy determination
- Standardized dosing protocols for mood disorders and cognitive enhancement
- Long-term safety studies including neurological and cardiovascular effects
- Mechanism validation studies in human populations
- Drug interaction studies with psychiatric and neurological medications
Emerging Applications
Investigational Uses:
- Treatment-resistant depression and anxiety disorders
- Age-related cognitive decline and dementia prevention
- Post-traumatic stress disorder and trauma-related conditions
- Attention deficit hyperactivity disorder management
- Chronic fatigue syndrome and fibromyalgia treatment
- Post-surgical cognitive recovery enhancement
Combination Therapies
Synergistic Research:
- Combination with other neuropeptides for enhanced cognitive effects
- Integration with traditional antidepressants for treatment-resistant cases
- Potential combination with neurostimulation therapies
- Synergistic effects with psychotherapy and behavioral interventions


Conclusion
PE-22-28 represents a promising novel therapeutic peptide with demonstrated potential in mood disorder treatment and cognitive enhancement through its unique TREK-1 channel inhibition mechanism. Its rapid-onset effects, excellent tolerability profile, and neuroplasticity-enhancing properties distinguish it from traditional psychiatric medications.
However, the current clinical evidence base remains predominantly preclinical, with limited human clinical data available. The lack of FDA approval, regulatory restrictions, and need for comprehensive clinical validation necessitate careful consideration and medical supervision for any therapeutic application.
Patients considering PE-22-28 therapy should engage in thorough discussions with qualified healthcare providers to weigh potential benefits against risks and explore evidence-based treatment alternatives. Future research will be critical in establishing the safety, efficacy, and appropriate clinical applications of PE-22-28 in human neuropsychiatric medicine. Until comprehensive clinical trials are completed, its use should remain limited to research settings under appropriate medical oversight.
PE-22-28 SCIENTIFIC
DATA SUMMARY
Disclaimer: This information is provided for educational purposes only and does not constitute medical advice. PE-22-28 is not approved by the FDA for human therapeutic use. Patients should consult with qualified healthcare providers before considering any peptide therapy.
The content reflects current scientific literature and regulatory status as of 2025.
