Pentadeca Arginate (PDA)
Pentadeca Arginate (PDA) is a synthetic pentadecapeptide that represents an advanced formulation of the well-established BPC-157, utilizing an arginate salt instead of the traditional acetate salt. This molecular modification enhances the peptide's stability, bioavailability, and therapeutic efficacy while maintaining the comprehensive healing properties that have made BPC-157 a cornerstone in regenerative medicine.
PDA demonstrates superior tissue healing capabilities through enhanced angiogenesis, accelerated collagen synthesis, and potent anti-inflammatory effects. Its unique formulation offers improved pharmacokinetic properties and potentially reduced side effects compared to its predecessor, making it unique among therapeutic peptides in the healing and regenerative medicine space.

Overview
PDA demonstrates enhanced sequence stability with the arginate modification, contributing to its superior therapeutic profile compared to traditional acetate formulations. The peptide shows improved metabolic resistance and enhanced bioavailability, with detectability extending beyond traditional BPC-157 formulations using standard analytical methods.

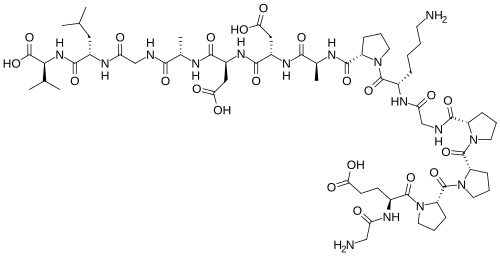
Chemical structure & Properties
- Molecular Formula: C62H98N16O22 (with arginate salt modification)
- Molecular Weight: Approximately 1419 Da
- Sequence: Based on BPC-157 pentadecapeptide with arginate salt enhancement
- Half-life: Enhanced stability compared to acetate formulation
- Stability: Superior resistance to enzymatic degradation, improved gastric acid tolerance
Mechanism of Action
PDA exerts its enhanced therapeutic effects through multiple interconnected molecular pathways:
Clinical Applications and
Research Evidence

Current Clinical Evidence
Safety Profile and Considerations
Regulatory Status and
Legal Considerations
FDA Status
- Classification: Investigational compound based on Category 2 bulk drug substance classification
- Approval Status: Not approved for human therapeutic use
- Compounding: Not available for commercial pharmaceutical compounding
- Regulatory Position: Insufficient evidence for safety determination of enhanced formulation
WADA Status
- Classification: Prohibited under S0: Non-Approved Substances
- Athletic Use: Banned in competitive sports
- Testing: Potentially detectable in anti-doping screenings
Legal Availability
- Commercial Status: Not legally available as prescription medication
- Market Presence: May be sold as "research chemicals" with enhanced formulation claims
- Quality Control: No regulatory oversight for purity or potency of arginate formulation
- Clinical Use: Limited to research settings and experimental protocols
Administration and Dosing
Considerations
The Paragon Method: Step-by-Step
Clinical Considerations
Important Notes:
- No FDA-approved dosing guidelines exist for human use of PDA
- Individual response may vary significantly with enhanced formulation
- Medical supervision strongly recommended due to enhanced potency
- Quality and purity of commercially available enhanced formulations not guaranteed

Priority Research Areas
- Large-scale human clinical trials for safety and efficacy determination of PDA
- Comparative studies with BPC-157 to quantify enhancement benefits
- Standardized dosing protocols for various clinical applications with arginate formulation
- Long-term safety studies including carcinogenicity assessment of enhanced formulation
- Drug interaction studies with commonly prescribed medications
- Mechanism elucidation of arginate-specific molecular targets and pathways
Emerging Applications
Research is investigating potential enhanced applications in:
- Traumatic brain injury and neuroprotection with superior outcomes
- Wound healing and post-surgical recovery with accelerated timelines
- Age-related degenerative conditions with enhanced therapeutic response
- Metabolic disorders and diabetes complications with improved management
- Cardiovascular protection with enhanced vascular benefits


Conclusion
Pentadeca Arginate represents a promising advancement in therapeutic peptide technology, building upon the established benefits of BPC-157 while offering enhanced stability, improved bioavailability, and potentially superior clinical outcomes through strategic molecular modification. Its enhanced mechanism of action through multiple molecular pathways offers theoretical advantages in regenerative medicine applications compared to traditional formulations.
However, the current evidence base remains predominantly theoretical and preclinical, with limited human clinical data specifically for the PDA formulation. The lack of FDA approval, potential safety concerns related to increased potency, and regulatory restrictions necessitate careful consideration and enhanced medical supervision for any therapeutic application.
Patients interested in PDA therapy should engage in thorough discussions with qualified healthcare providers to weigh potential enhanced benefits against unknown risks and explore evidence-based treatment alternatives. Future research will be critical in establishing the safety, efficacy, and appropriate clinical applications of PDA in human medicine. Until comprehensive clinical trials are completed, its use should remain limited to research settings and experimental protocols under appropriate medical oversight.
PDA SCIENTIFIC
DATA SUMMARY
Disclaimer: This information is provided for educational purposes only and does not constitute medical advice. PDA is not approved by the FDA for human therapeutic use. Patients should consult with qualified healthcare providers before considering any enhanced peptide therapy.
The content reflects current scientific literature and regulatory status as of 2025, with theoretical considerations based on molecular modifications to established compounds.
