PT-141
PT-141, also known as Bremelanotide, is a synthetic cyclic heptapeptide derived from alpha-melanocyte-stimulating hormone (α-MSH) that functions as a selective melanocortin receptor agonist. Originally developed as a derivative of the tanning peptide Melanotan II, PT-141 represents a novel approach to sexual dysfunction treatment by targeting central nervous system pathways rather than peripheral vascular mechanisms. Through selective activation of melanocortin-3 and melanocortin-4 receptors (MC3R/MC4R) in the hypothalamus and limbic regions, PT-141 enhances sexual desire and arousal through neurogenic pathways.
This peptide has gained FDA approval as Vyleesi for the treatment of hypoactive sexual desire disorder (HSDD) in premenopausal women and demonstrates significant efficacy in clinical studies for both male and female sexual dysfunction. PT-141's unique central mechanism of action, rapid onset of effects, and distinct pharmacological profile distinguish it from traditional sexual dysfunction treatments, making it a valuable therapeutic option for patients unresponsive to conventional therapies.
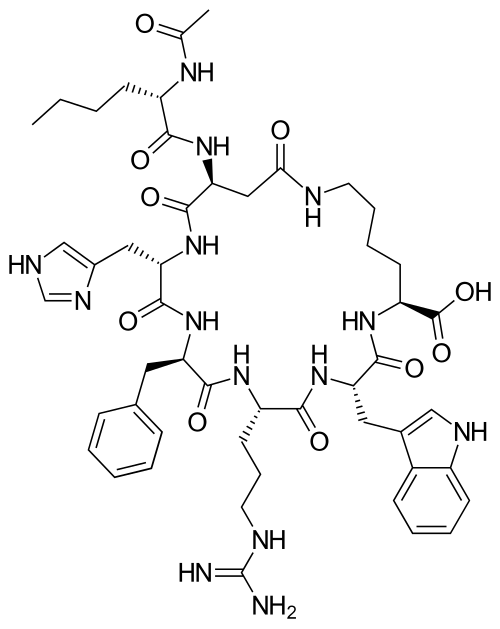

Overview
PT-141 demonstrates high water solubility and excellent bioavailability via subcutaneous and intranasal administration routes. The peptide exhibits selective receptor binding affinity for melanocortin receptors, with minimal cross-reactivity to other receptor systems. It is metabolized primarily through hepatic pathways and eliminated via renal excretion within 24 hours post-administration.

Chemical structure & Properties
- Molecular Formula: C50H68N14O10
- Molecular Weight: 1025.16 Da
- Sequence: Cyclic heptapeptide: Ac-Nle-cyclo[Asp-His-D-Phe-Arg-Trp-Lys]-OH
- Half-life: Approximately 2.5 hours (plasma elimination)
- Stability: Stable synthetic peptide with resistance to enzymatic degradation
Mechanism of Action
PT-141 exerts its therapeutic effects through selective activation of melanocortin receptors in the central nervous system:
Clinical Applications and
Research Evidence

Current Clinical Evidence
Safety Profile and Considerations
Regulatory Status and
Legal Considerations
FDA Status
- Classification: Prescription medication (Vyleesi)
- Approval Status: Approved for HSDD in premenopausal women (June 2019)
- Indication: Treatment of hypoactive sexual desire disorder
- Regulatory Position: Full FDA approval with established safety and efficacy profile
International Status
- European Medicines Agency: Under review for potential approval
- Other Jurisdictions: Regulatory submissions in progress in multiple countries
- Off-Label Use: Permitted under physician supervision for approved indications
Legal Availability
- Commercial Status: Legally available as prescription medication (Vyleesi)
- Market Presence: Distributed through specialty pharmacies
- Quality Control: Full pharmaceutical manufacturing and quality standards
- Clinical Use: Approved for clinical use under medical supervision
Administration and Dosing
Considerations
The Paragon Method: Step-by-Step
Administration Routes
- Subcutaneous injection: FDA-approved route with optimal bioavailability
- Intranasal administration: Investigational route with rapid absorption
- Timing considerations: 45-60 minute onset, 4-8 hour duration of action
- Patient education: Proper injection technique and site rotation
Clinical Considerations
Important Guidelines:
- Individual dose optimization essential for balancing efficacy and tolerability
- Pre-medication with anti-emetics may reduce nausea in sensitive patients
- Medical supervision recommended during initial dosing and titration
- Patient counseling regarding realistic expectations and timing of effects

Priority Research Areas
Expanded Clinical Applications:
- Male erectile dysfunction FDA approval studies
- Postmenopausal women with sexual dysfunction
- Sexual dysfunction in special populations (diabetes, spinal cord injury)
- Combination therapy protocols with other sexual health treatments
Long-Term Studies:
- Extended safety evaluation beyond current 24-week data
- Chronic use effects on melanocortin system function
- Long-term efficacy maintenance and potential tolerance development
- Cardiovascular safety in extended treatment protocols
Emerging Applications
Research is investigating potential applications in:
- Female sexual arousal disorder beyond HSDD
- Post-SSRI sexual dysfunction syndrome
- Menopausal sexual dysfunction treatment
- Sexual dysfunction in cancer survivors
- Relationship and couples therapy enhancement


Conclusion
PT-141 (Bremelanotide) represents a significant advancement in sexual dysfunction treatment, offering the first FDA-approved therapy specifically targeting the neurological basis of sexual desire through melanocortin receptor activation. Its unique mechanism of action provides therapeutic benefits for female hypoactive sexual desire disorder with proven efficacy and an established safety profile.
The peptide's central nervous system-based approach offers advantages over peripheral treatments, particularly for patients with psychogenic or neurogenic sexual dysfunction components. The FDA approval for female HSDD establishes PT-141 as a legitimate medical treatment with full regulatory oversight and quality standards.
Healthcare providers considering PT-141 therapy should conduct comprehensive patient assessments, implement appropriate dosing strategies, and provide thorough patient education regarding proper use and expected effects. The medication's dose-dependent side effects require careful individual optimization to achieve optimal therapeutic outcomes while minimizing adverse effects.
Future research expanding PT-141's approved indications and establishing optimal treatment protocols will likely broaden its clinical utility, offering patients effective, scientifically-validated treatment for various forms of sexual dysfunction through targeted neurological enhancement.
PT-141 SCIENTIFIC
DATA SUMMARY
Disclaimer: This information is provided for educational purposes only and does not constitute medical advice. PT-141 (Vyleesi) is FDA-approved for specific indications and requires prescription and medical supervision. Patients should consult with qualified healthcare providers before considering any peptide therapy.
The content reflects current scientific literature and regulatory status as of 2025.
