Selank
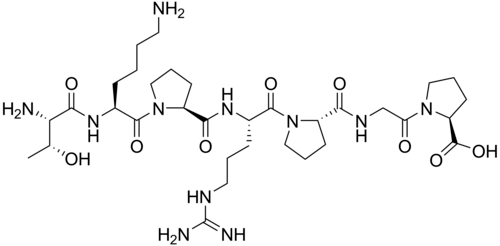
Selank is a synthetic heptapeptide consisting of seven amino acids (Thr-Lys-Pro-Arg-Pro-Gly-Pro) derived from the naturally occurring immunoregulatory peptide tuftsin. Originally developed at the Institute of Molecular Genetics of the Russian Academy of Sciences in cooperation with the V.V. Zakusov Research Institute of Pharmacology, Selank represents a metabolically stable analog of the tuftsin tetrapeptide (Thr-Lys-Pro-Arg) found in human immunoglobulin G heavy chains.
This peptide has gained considerable attention in neuropsychopharmacology due to its demonstrated anxiolytic and nootropic effects in both preclinical and clinical studies, particularly in anxiety management, cognitive enhancement, and mood regulation. Selank exhibits remarkable stability compared to its endogenous counterpart and maintains bioactivity through modulation of GABAergic, dopaminergic, and serotonergic neurotransmitter systems, making it unique among therapeutic peptides.

Overview
Selank demonstrates no sequence homology with other known anxiolytic compounds, contributing to its unique therapeutic profile. The peptide is metabolized rapidly in the liver and eliminated through renal excretion, with complete clearance from circulation within 10 minutes post-administration using sensitive detection methods.

Chemical structure & Properties
- Molecular Formula: C37H51N11O9
- Molecular Weight: 751.86 g/mol
- Sequence: Thr-Lys-Pro-Arg-Pro-Gly-Pro (TKPRPGP)
- Half-life: Approximately 2-3 minutes in circulation (rapid hepatic metabolism)
- Stability: Enhanced metabolic stability compared to tuftsin due to C-terminal extension
Mechanism of Action
Selank exerts its therapeutic effects through multiple interconnected molecular pathways:
Clinical Applications and
Research Evidence

Current Clinical Evidence
Safety Profile and Considerations
Regulatory Status and
Legal Considerations
FDA Status
- Classification: Not approved for human therapeutic use
- Category: Classified under non-approved substances (2024)
- Compounding: Prohibited for commercial pharmaceutical compounding
- Research Use: Available as research chemical only
International Status
- Russia: Approved for treatment of generalized anxiety disorder
- CIS Countries: Limited approval for anxiety disorders
- European Union: Not approved for therapeutic use
- Other Countries: Generally classified as research chemical
WADA Status
- Classification: Not specifically listed on the Prohibited List (2025)
- Athletic Use: Regulatory status may change; caution advised
- Testing: Detection methods available for anti-doping purposes
Legal Availability
- Commercial Status: Not legally available as prescription medication in most countries
- Market Presence: Sold as research chemicals or investigational compounds
- Quality Control: No regulatory oversight for purity or potency in research markets
- Clinical Use: Limited to research settings and experimental protocols
Administration and Dosing
Considerations
Administration Routes:
- Intranasal spray (most studied route)
- Subcutaneous injection
- Intramuscular injection (limited data)
Clinical Considerations
Important Notes:
- No FDA-approved dosing guidelines exist for human use
- Individual response varies significantly based on genetic factors
- Medical supervision recommended for any therapeutic application
- Cycling protocols may be necessary to prevent receptor desensitization
Formulation Concerns:
- Quality and purity of commercially available products not guaranteed
- Compounding pharmacy standards vary significantly
- Storage requirements critical for maintaining peptide stability

Priority Research Areas
- Large-scale international clinical trials for safety and efficacy determination
- Long-term safety studies including effects on GABAergic system function
- Standardized dosing protocols for various clinical applications
- Pharmacokinetic studies in diverse populations
- Drug interaction studies with commonly prescribed psychoactive medications
Emerging Applications
Research is investigating potential applications in:
- Post-traumatic stress disorder (PTSD) treatment
- Attention deficit hyperactivity disorder (ADHD) management
- Substance abuse disorder recovery support
- Age-related cognitive decline prevention
- Chronic fatigue syndrome and fibromyalgia
Mechanistic Studies
- Detailed characterization of GABAergic allosteric modulation
- Investigation of BDNF-mediated neuroprotective mechanisms
- Elucidation of immune system interactions
- Study of gene expression changes in target brain regions


Conclusion
Selank represents a promising therapeutic peptide with demonstrated anxiolytic and nootropic properties in both preclinical and clinical studies. Its unique mechanism of action through GABAergic system modulation, combined with neuroprotective and cognitive-enhancing effects, offers potential advantages over traditional anxiolytic medications.
However, the current evidence base remains predominantly from Russian research institutions, with limited international validation and long-term safety data. The lack of FDA approval, potential safety concerns regarding immunogenicity, and regulatory restrictions necessitate careful consideration and medical supervision for any therapeutic application.
The peptide's favorable side effect profile compared to benzodiazepines, absence of dependence potential, and additional cognitive benefits make it an intriguing candidate for further research. Patients interested in Selank therapy should engage in thorough discussions with qualified healthcare providers to weigh potential benefits against risks and explore evidence-based treatment alternatives.
Future international clinical trials will be critical in establishing the safety, efficacy, and appropriate clinical applications of Selank in human medicine. Until comprehensive clinical validation is completed, its use should remain limited to research settings and experimental protocols under appropriate medical oversight.
Selank Scientific
DATA SUMMARY
Disclaimer: This information is provided for educational purposes only and does not constitute medical advice. Selank is not approved by the FDA for human therapeutic use. Patients should consult with qualified healthcare providers before considering any peptide therapy.
The content reflects current scientific literature and regulatory status as of 2025.
