Semaglutide
Semaglutide is a synthetic glucagon-like peptide-1 (GLP-1) receptor agonist consisting of a 31-amino acid sequence with 94% homology to human GLP-1, modified to enhance stability and extend half-life. Originally developed from the naturally occurring incretin hormone GLP-1, semaglutide represents a significant advancement in metabolic health management through targeted modulation of glucose homeostasis and appetite regulation. The peptide incorporates strategic modifications including albumin binding properties and dipeptidyl peptidase-4 (DPP-4) resistance to achieve once-weekly dosing.
This medication has gained widespread clinical adoption due to its demonstrated superior efficacy in glycemic control and substantial weight reduction through multiple mechanisms including glucose-dependent insulin secretion, suppressed glucagon release, delayed gastric emptying, and central appetite regulation. Semaglutide exhibits remarkable clinical effectiveness with average weight loss of 15-20% in obesity management and comprehensive cardiovascular benefits, making it a cornerstone therapy in modern diabetes and obesity treatment.

Overview
Semaglutide demonstrates 94% sequence homology with endogenous human GLP-1 but incorporates critical structural modifications to overcome the rapid degradation that limits native GLP-1 therapeutic utility. The peptide is administered subcutaneously and distributed systemically through albumin binding, with elimination primarily through proteolytic degradation and renal excretion over approximately one week.
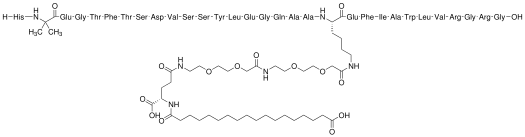
Chemical structure & Properties
- Molecular Formula: C187H291N45O59
- Molecular Weight: 4113.58 Da
- Sequence: Modified 31-amino acid GLP-1 analog with albumin-binding modifications
- Half-life: Approximately 165 hours (7 days) enabling once-weekly administration
- Stability: Enhanced through albumin binding and DPP-4 resistance modifications
Mechanism of Action
Semaglutide exerts its therapeutic effects through comprehensive GLP-1 receptor activation across multiple organ systems:
Clinical Applications and
Research Evidence

Current Clinical Evidence
Safety Profile and Considerations
Regulatory Status and
Legal Considerations
FDA Status
- Classification: Prescription medication with multiple approved formulations
- Approval Status: Fully approved for type 2 diabetes (Ozempic) and obesity management (Wegovy)
- Indications: Type 2 diabetes mellitus and chronic weight management
- Regulatory Position: Comprehensive safety and efficacy profile established through clinical trials
International Status
- European Medicines Agency: Approved for both diabetes and weight management indications
- Other Jurisdictions: Widely approved internationally with consistent regulatory decisions
- Clinical Guidelines: Incorporated into major medical society treatment recommendations
Legal Availability
- Commercial Status: Legally available as prescription medication through standard channels
- Market Presence: Multiple formulations including subcutaneous and oral preparations
- Quality Control: Full pharmaceutical manufacturing standards and regulatory oversight
- Clinical Use: Approved for clinical use under appropriate medical supervision
Administration and Dosing
Considerations
The Paragon Method: Step-by-Step
Alternative Dosing Strategies
Conservative Titration Approach:
- Rationale: Minimize gastrointestinal side effects and optimize tolerability
- Protocol: Slower dose escalation with extended periods at lower doses
- Monitoring: Individual response assessment and symptom-guided titration
- Applications: Patients with high sensitivity to GLP-1 agonist effects
Administration Routes
- Subcutaneous injection: Primary FDA-approved administration method
- Oral formulation: Rybelsus available for diabetes management with absorption enhancer
- Timing flexibility: Once-weekly dosing any day of the week, consistent timing preferred
- Storage requirements: Refrigerated storage until use, limited room temperature stability
Clinical Considerations
Important Guidelines:
- Medical supervision essential for appropriate patient selection and monitoring
- Individual dose optimization based on efficacy, tolerability, and treatment goals
- Comprehensive lifestyle modification program integration for optimal outcomes
- Storage requirements: Refrigerated storage until use, limited room temperature stability

Priority Research Areas
Long-Term Safety Studies:
- Extended cardiovascular outcomes data beyond current follow-up periods
- Rare adverse event identification through post-marketing surveillance
- Long-term effects on bone health, kidney function, and other organ systems
- Pregnancy safety data and reproductive health effects
Expanded Clinical Applications:
- Pediatric and adolescent obesity management protocols
- Prevention applications in pre-diabetes and metabolic syndrome
- Combination therapy optimization with other metabolic agents
- Precision medicine approaches based on individual biomarkers
Emerging Applications
Research is investigating potential applications in:
- Non-alcoholic fatty liver disease treatment and prevention
- Polycystic ovary syndrome management
- Sleep apnea improvement through weight reduction
- Cognitive function enhancement through metabolic optimization
- Recovery optimization in post-surgical and rehabilitation settings


Conclusion
Semaglutide represents a significant advancement in metabolic health management, offering superior efficacy in type 2 diabetes control and obesity treatment through comprehensive GLP-1 receptor agonism. Clinical evidence from large-scale trials demonstrates average weight loss of 15-20% and substantial cardiovascular benefits with a well-established safety profile.The medication's ability to simultaneously address glucose homeostasis, weight management, and cardiovascular protection through once-weekly dosing positions it as a cornerstone therapy in modern metabolic medicine. However, optimal outcomes require integration with comprehensive lifestyle modifications and careful medical supervision.
Healthcare providers should implement individualized treatment plans with appropriate patient selection, gradual dose titration, and ongoing monitoring to maximize therapeutic benefits while minimizing gastrointestinal side effects. The established FDA approval for both diabetes (Ozempic) and obesity management (Wegovy) provides regulatory confidence for clinical use.
Future research will likely expand approved indications and optimize treatment protocols, but current evidence establishes semaglutide as a highly effective therapeutic option for patients requiring significant metabolic health intervention under appropriate medical supervision.
SEMAGLUTIDE SCIENTIFIC
DATA SUMMARY
Disclaimer: This information is provided for educational purposes only and does not constitute medical advice. Semaglutide requires prescription and medical supervision. Patients should consult with qualified healthcare providers before considering any GLP-1 receptor agonist therapy.
The content reflects current scientific literature and regulatory status as of 2025.
