Semax
Semax is a synthetic heptapeptide derived from the N-terminal fragment of adrenocorticotropic hormone (ACTH 4-10), specifically engineered for neuroprotective and cognitive enhancement applications. Developed through systematic peptide modification research in Russia, Semax (Met-Glu-His-Phe-Pro-Gly-Pro) represents a breakthrough in rational peptide design, optimized to enhance cognitive function while providing robust protection against neurological injury without the hormonal side effects of native ACTH.
This peptide has gained considerable attention in neurology and cognitive medicine due to its demonstrated multifaceted benefits in stroke recovery, traumatic brain injury rehabilitation, and cognitive performance optimization through comprehensive modulation of neurotransmitter systems, gene expression regulation, and neuroprotective pathways. Semax exhibits excellent CNS penetration via intranasal administration and maintains bioactivity across various physiological conditions, making it unique among neuroprotective peptides.

Overview
Semax demonstrates superior stability compared to native ACTH fragments through strategic amino acid modifications that resist enzymatic degradation. The peptide exhibits excellent water solubility and high intranasal bioavailability with rapid CNS penetration. It is metabolized primarily through peptidase activity and eliminated via renal excretion, with detectability in plasma for several hours post-administration.

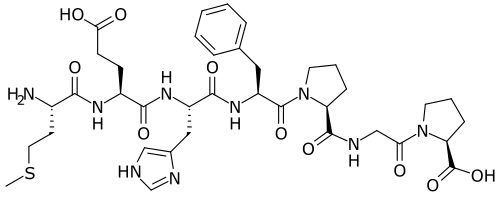
Chemical structure & Properties
- Molecular Formula: C37H51N9O10S
- Molecular Weight: 813.93 Da
- Sequence: Met-Glu-His-Phe-Pro-Gly-Pro (ACTH 4-10 analog)
- Half-life: Approximately 1-2 hours (plasma elimination)
- Stability: Enhanced resistance to peptidase degradation through structural modifications
Mechanism of Action
Semax exerts its therapeutic effects through multiple interconnected neurobiological pathways:
Clinical Applications and
Research Evidence

Current Clinical Evidence
Safety Profile and Considerations
Regulatory Status and
Legal Considerations
International Regulatory Status
- Classification: Research peptide/investigational compound in most jurisdictions
- Approval Status: Not approved for human therapeutic use in the United States or EU
- Russian Federation: Approved for clinical use in specific neurological applications
- Regulatory Position: Limited regulatory oversight outside of Russia
Legal Availability
- Commercial Status: Available as research chemical through specialized suppliers
- Market Presence: Sold for research purposes without therapeutic claims
- Quality Control: Variable quality and purity standards across different suppliers
- Clinical Use: Limited to research settings and specialized medical practices
Research Applications
- Academic Research: Widely used in neuroscience research protocols
- Clinical Investigation: Ongoing studies in various neurological applications
- International Interest: Growing research interest in neuroprotective applications
Administration and Dosing
Considerations
The Paragon Method: Step-by-Step
Administration Routes
- Intranasal delivery: Most common route with excellent CNS penetration
- Subcutaneous injection: Alternative route for consistent systemic delivery
- Timing considerations: Morning administration optimal for cognitive benefits
- Cycle protocols: Periodic breaks recommended to prevent adaptation
Clinical Considerations
Important Guidelines:
- Medical supervision recommended for all therapeutic applications
- Individual response assessment and dose optimization essential
- Quality verification important due to variable commercial preparations
- Integration with comprehensive neurological care for optimal outcomes

Priority Research Areas
Clinical Trial Development:
- Large-scale randomized controlled trials for stroke recovery applications
- Systematic evaluation of cognitive enhancement efficacy in healthy populations
- Safety and efficacy studies in pediatric neurodevelopmental disorders
- Long-term safety assessment in extended treatment protocols
Mechanistic Studies:
- Detailed characterization of gene expression modulation patterns
- Investigation of optimal dosing strategies for different clinical applications
- Biomarker development for treatment response prediction
- Combination therapy protocols with other neuroprotective agents
Emerging Applications
Research is investigating potential applications in:
- Alzheimer's disease and other neurodegenerative conditions
- Post-traumatic stress disorder and anxiety-related cognitive impairment
- Age-related cognitive decline prevention and treatment
- Enhancement of learning and memory in educational and training contexts
- Recovery optimization in post-surgical and rehabilitation settings


Conclusion
Semax represents a significant advancement in neuropeptide therapeutics, offering comprehensive neuroprotective and cognitive-enhancing properties through its unique ACTH-derived structure and multifaceted mechanism of action. The substantial preclinical evidence and emerging clinical data support its therapeutic potential across diverse neurological applications, from acute stroke treatment to cognitive optimization.
The peptide's exceptional safety profile, multiple administration routes, and comprehensive mechanism of action position it as a valuable tool in neurological medicine. Its ability to modulate gene expression, enhance neuroprotection, and optimize cognitive function simultaneously makes it particularly valuable for complex neurological conditions requiring multifaceted therapeutic approaches.
Healthcare providers considering Semax should conduct thorough patient assessments and implement appropriate monitoring protocols. The peptide's excellent tolerability and lack of significant adverse effects make it suitable for both acute interventions and long-term cognitive optimization, though the current regulatory status limits its clinical availability outside of research settings.
Future clinical development should focus on establishing standardized dosing protocols and conducting large-scale efficacy trials to support broader regulatory approval and clinical implementation.
SEMAX SCIENTIFIC
DATA SUMMARY
Disclaimer: This information is provided for educational purposes only and does not constitute medical advice. Semax is not approved by the FDA for human therapeutic use. Patients should consult with qualified healthcare providers before considering any peptide therapy.
The content reflects current scientific literature and regulatory status as of 2025.
