Sermorelin
Sermorelin is a synthetic 29-amino acid peptide analog of the naturally occurring growth hormone-releasing hormone (GHRH 1-29), specifically designed to stimulate endogenous growth hormone (GH) production from the anterior pituitary gland. Originally developed as the biologically active N-terminal fragment of human GHRH, sermorelin represents a physiologically appropriate approach to growth hormone optimization that preserves natural pulsatile secretion patterns and regulatory feedback mechanisms.
This peptide has gained considerable attention in endocrinology and age management medicine due to its demonstrated efficacy in treating growth hormone deficiency while maintaining physiological safety through preservation of the body's natural regulatory systems. Sermorelin exhibits excellent bioavailability via subcutaneous administration and stimulates growth hormone release without disrupting natural circadian rhythms or feedback inhibition, making it unique among growth hormone-related therapeutics.

Overview
Sermorelin demonstrates excellent water solubility and rapid absorption following subcutaneous injection. The peptide exhibits high affinity for GHRH receptors on pituitary somatotroph cells and is metabolized primarily through enzymatic degradation. Despite its short plasma half-life, sermorelin's biological effects persist through sustained growth hormone release for several hours post-administration.

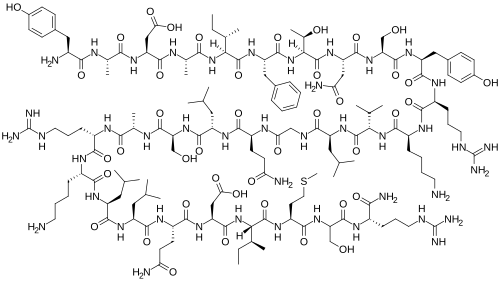
Chemical structure & Properties
- Molecular Formula: C149H246N44O42S
- Molecular Weight: 3357.96 Da
- Sequence: N-terminal 29 amino acids of human GHRH (1-29)
- Half-life: 8-12 minutes (rapid plasma clearance)
- Stability: Requires proper refrigeration and handling due to peptide nature
Mechanism of Action
Sermorelin exerts its therapeutic effects through specific stimulation of the growth hormone-releasing hormone receptor pathway:
Clinical Applications and
Research Evidence

Current Clinical Evidence
Safety Profile and Considerations
Regulatory Status and
Legal Considerations
FDA Status
- Classification: Prescription medication approved for growth hormone deficiency
- Approval Status: Fully approved for pediatric and adult growth hormone deficiency
- Indications: Growth hormone deficiency diagnosis and treatment
- Regulatory Position: Established safety and efficacy profile through clinical trials
Clinical Practice Status
- Endocrinology Guidelines: Recognized therapy in clinical practice guidelines
- Anti-Aging Medicine: Widely accepted in age management medical practice
- Pediatric Endocrinology: Standard diagnostic and therapeutic tool for GH deficiency
Legal Availability
- Commercial Status: Legally available as prescription medication
- Market Presence: Available through licensed healthcare providers and specialty pharmacies
- Quality Control: Full pharmaceutical manufacturing standards and regulatory oversight
- Clinical Use: Approved for clinical use under appropriate medical supervision
Administration and Dosing
Considerations
The Paragon Method: Step-by-Step
Administration Routes
- Subcutaneous injection: Primary FDA-approved route with predictable absorption
- Sublingual tablets: Alternative formulation for patients preferring non-injection routes
- Timing considerations: Bedtime administration optimal for physiological GH release
- Storage requirements: Refrigeration required for peptide stability
Clinical Considerations
Important Guidelines:
- Medical supervision essential for appropriate patient selection and monitoring
- Regular IGF-1 level monitoring to guide dose optimization
- Comprehensive endocrine evaluation before treatment initiation
- Integration with lifestyle modifications for optimal therapeutic outcomes

Priority Research Areas
Clinical Applications:
- Long-term safety studies in extended treatment protocols
- Optimal dosing strategies for various therapeutic applications
- Combination therapy protocols with other regenerative treatments
- Biomarker development for treatment response prediction
Novel Applications:
- Expansion of oncological applications and combination cancer therapy protocols
- Anti-aging and longevity research applications
- Metabolic disorder treatment and prevention applications
- Athletic performance and recovery enhancement studies
Emerging Applications
Research is investigating potential applications in:
- Age-related muscle wasting and sarcopenia prevention
- Metabolic syndrome and insulin resistance management
- Bone density optimization and osteoporosis prevention
- Cognitive function enhancement and neuroprotection
- Wound healing and post-surgical recovery acceleration


Conclusion
Sermorelin represents a physiologically sound approach to growth hormone optimization through stimulation of endogenous GH production while preserving natural regulatory mechanisms. Its FDA approval for both pediatric and adult growth hormone deficiency, combined with extensive clinical experience, establishes it as a safe and effective alternative to direct growth hormone replacement therapy.
The peptide's excellent safety profile and ability to maintain physiological GH release patterns make it particularly valuable for long-term therapy. Healthcare providers should conduct comprehensive assessments and implement appropriate monitoring protocols, including regular IGF-1 level evaluation and clinical response assessment.
Future research may expand clinical applications, but current evidence establishes sermorelin as a proven therapeutic option for growth hormone deficiency under appropriate medical supervision.
SERMORELIN SCIENTIFIC
DATA SUMMARY
Disclaimer: This information is provided for educational purposes only and does not constitute medical advice. Sermorelin requires prescription and medical supervision. Patients should consult with qualified healthcare providers before considering any growth hormone-related therapy.
The content reflects current scientific literature and regulatory status as of 2025.
