SS-31 (Elamipretide)
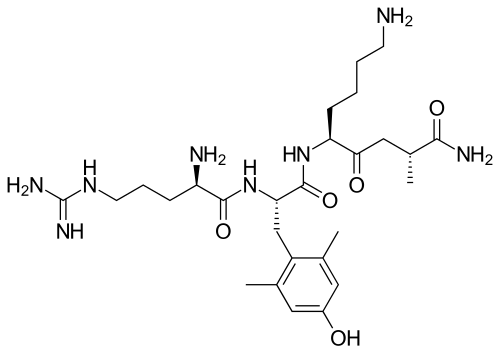
SS-31, also known as Elamipretide, is a synthetic tetrapeptide (Dimethyltyrosine-D-Arginine-Dimethyltyrosine-Lysine-NH2) specifically designed to target mitochondrial dysfunction through selective binding to cardiolipin, a unique phospholipid found exclusively in the inner mitochondrial membrane. Originally developed as a mitochondria-penetrating peptide, SS-31 represents a novel therapeutic approach addressing fundamental cellular energetic dysfunction underlying numerous age-related diseases and pathological conditions.
This peptide has gained considerable attention in mitochondrial medicine due to its demonstrated ability to stabilize cardiolipin, optimize electron transport chain organization, and enhance cellular energy production in preclinical and clinical studies. SS-31 exhibits selective mitochondrial accumulation and maintains bioactivity through its unique cationic structure, making it distinctive among therapeutic peptides targeting cellular energetics.

Overview
SS-31 demonstrates excellent water solubility and selective mitochondrial uptake due to its cationic properties. The peptide's unique structure enables penetration of mitochondrial membranes and selective accumulation within mitochondria based on concentration gradients and tissue mitochondrial density. It is eliminated primarily through renal excretion with minimal hepatic metabolism.

Chemical structure & Properties
- Molecular Formula: C20H32N8O5
- Molecular Weight: 464.52 Da
- Sequence: Dimethyltyrosine-D-Arginine-Dimethyltyrosine-Lysine-NH2
- Half-life: 1-2 hours (plasma elimination)
- Stability: Stable peptide structure with enhanced membrane penetration properties
Mechanism of Action
SS-31 exerts its therapeutic effects through highly specific mitochondrial targeting and cardiolipin stabilization:
Clinical Applications and
Research Evidence

Current Clinical Evidence
Safety Profile and Considerations
Regulatory Status and
Legal Considerations
Clinical Development Status
- Classification: Investigational drug in clinical development
- Approval Status: Not approved for routine therapeutic use
- Clinical Trials: Multiple ongoing studies for various indications
- Regulatory Position: Investigational new drug (IND) status for clinical research
Research Applications
- Academic Studies: Extensive use in mitochondrial research protocols
- Clinical Trials: Available through approved research protocols
- Orphan Drug Status: Designated for certain rare mitochondrial diseases
Legal Availability
- Commercial Status: Not available as prescription medication
- Research Access: Available through clinical trial participation
- Quality Control: Manufacturing under clinical trial standards
- Clinical Use: Limited to approved research and clinical trial settings
Administration and Dosing
Considerations
The Paragon Method: Step-by-Step
Administration Routes
- Intravenous infusion: Maximum bioavailability for acute mitochondrial dysfunction
- Subcutaneous injection: Convenient outpatient administration with excellent absorption
- Timing considerations: Can be administered any time of day
- Monitoring requirements: Regular assessment of energy levels and clinical response
Clinical Considerations
Important Guidelines:
- Medical supervision essential due to investigational status
- Individual response assessment and dose optimization required
- Integration with comprehensive mitochondrial evaluation protocols
- Quality verification important for clinical research applications

Priority Research Areas
Clinical Development:
- Large-scale randomized controlled trials for various mitochondrial diseases
- Dose optimization studies for different clinical conditions and patient populations
- Long-term safety evaluation in human clinical use
- Biomarker development for mitochondrial function assessment and treatment response
Mechanistic Studies:
- Detailed characterization of cardiolipin interaction mechanisms
- Investigation of tissue-specific mitochondrial targeting and accumulation patterns
- Combination therapy protocols with other mitochondrial-supporting agents
- Precision medicine approaches based on individual mitochondrial function profiles
Emerging Applications
Research is investigating potential applications in:
- Neurodegenerative diseases including Alzheimer's and Parkinson's disease
- Age-related muscle wasting and sarcopenia
- Diabetic complications and metabolic dysfunction
- Exercise performance enhancement and recovery optimization
- Longevity medicine and healthspan extension


Conclusion
SS-31 represents a groundbreaking advancement in mitochondrial medicine, offering targeted therapeutic intervention for fundamental cellular energetic dysfunction underlying numerous diseases and aging processes. Its unique mechanism of cardiolipin stabilization and mitochondrial optimization provides unprecedented therapeutic potential across diverse medical applications.
The substantial preclinical evidence demonstrates SS-31's therapeutic efficacy in cardiovascular, renal, and neurological applications with an exceptional safety profile. However, the current evidence base remains predominantly preclinical, requiring comprehensive human clinical trials for regulatory approval and clinical implementation.
Healthcare providers should approach SS-31 with understanding of its investigational status and the need for appropriate clinical trial participation or research protocols. The peptide's innovative mitochondrial targeting mechanism and proven preclinical efficacy position it as a promising therapeutic tool for conditions rooted in mitochondrial dysfunction.
Future clinical development will be critical in establishing SS-31's role in human medicine and optimizing treatment protocols for various mitochondrial-related conditions under appropriate medical oversight.
TB-500 SCIENTIFIC
DATA SUMMARY
Disclaimer: This information is provided for educational purposes only and does not constitute medical advice. SS-31 is an investigational drug not approved for routine therapeutic use. Patients interested in SS-31 should consult with qualified healthcare providers about clinical trial opportunities.
The content reflects current scientific literature and regulatory status as of 2025.
