TB-500
TB-500 (Thymosin Beta-4 acetate) is a synthetic 43-amino acid peptide derived from the naturally occurring thymosin beta-4 protein, which plays crucial roles in cellular migration, angiogenesis, and tissue regeneration. Originally identified in the thymus gland, thymosin beta-4 is one of the most abundant intracellular proteins in mammalian cells and serves as a critical regulator of actin polymerization and cellular motility. TB-500 is distinguished from endogenous thymosin beta-4 by its N-terminal acetylation, which enhances stability and biological activity.
This synthetic peptide has gained considerable attention in regenerative medicine due to its demonstrated ability to promote wound healing, reduce inflammation, and enhance tissue repair across multiple organ systems in preclinical models. TB-500 exhibits unique properties in promoting angiogenesis, cellular migration, and tissue remodeling through its primary mechanism of actin regulation, making it a subject of intensive research for therapeutic applications in wound healing, cardiovascular repair, and musculoskeletal injuries.
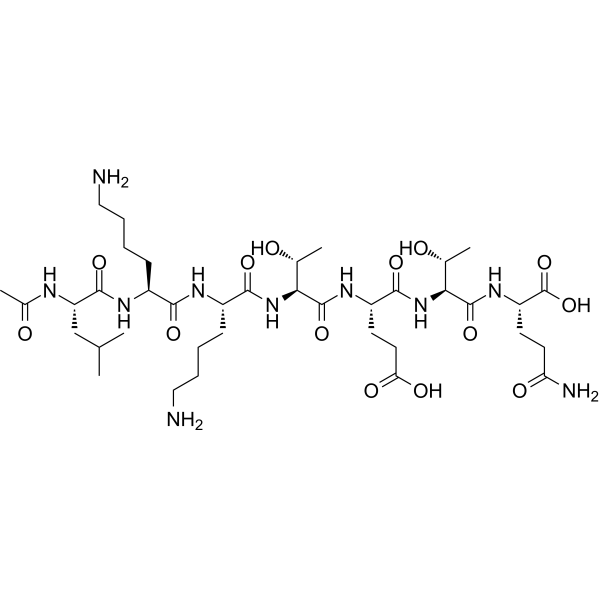

Overview
TB-500 demonstrates remarkable resistance to proteolytic degradation and maintains activity across various physiological pH ranges, contributing to its therapeutic potential. The peptide exhibits high systemic bioavailability via subcutaneous injection and distributes widely to tissues with high metabolic activity and injury sites.

Chemical structure & Properties
- Molecular Formula: C212H350N56O78S
- Molecular Weight: 4,963 Da
- Sequence: 43 amino acids with N-terminal acetylation
- Half-life: Approximately 2.5-3 hours following subcutaneous administration
- Stability: Stable at room temperature in lyophilized form, requires refrigeration when reconstituted
Mechanism of Action
TB-500 exerts its therapeutic effects through multiple interconnected cellular and molecular mechanisms:
Clinical Applications and
Research Evidence

Current Clinical Evidence
Safety Profile and Considerations
Regulatory Status and
Legal Considerations
FDA Status
- Classification: Category 2 bulk drug substance (2023 FDA guidance document)
- Approval Status: Not approved for human therapeutic use in any indication
- Compounding: Prohibited for commercial pharmaceutical compounding by FDA regulation
- Regulatory Position: Insufficient evidence for safety and efficacy determination
WADA Status
- Classification: Prohibited under S0: Non-Approved Substances category
- Athletic Use: Banned in competitive sports and subject to anti-doping penalties
- Testing: Detectable in anti-doping screening protocols and enforcement testing
Legal Availability
- Commercial Status: Not legally available as FDA-approved prescription medication
- Market Presence: Available through research chemical suppliers without regulatory oversight
- Quality Control: No regulatory oversight for purity, potency, or sterility standards
- Clinical Use: Limited to research institutions and experimental protocols under appropriate oversight
Administration and Dosing
Considerations
The Paragon Method: Step-by-Step
Clinical Considerations
Important Guidelines:
- No FDA-approved dosing guidelines exist for human therapeutic use
- Individual response variability significant requiring personalized approach
- Medical supervision essential for any therapeutic application or research use
- Quality and purity of commercially available products not guaranteed or regulated

Priority Research Areas
Clinical Development:
- Large-scale randomized controlled trials for safety and efficacy determination
- Standardized dosing protocols for various clinical applications and patient populations
- Long-term safety studies including carcinogenicity and reproductive toxicity assessment
- Biomarker development for treatment response prediction and monitoring
Mechanistic Studies:
- Detailed characterization of actin regulation and cellular migration mechanisms
- Investigation of optimal combination therapy protocols with other regenerative treatments
- Drug interaction studies with commonly prescribed medications
- Precision medicine approaches based on individual patient characteristics
Emerging Applications
Research is investigating potential applications in:
- Traumatic brain injury recovery and neuroprotection
- Stroke rehabilitation and neurological recovery enhancement
- Neurodegenerative disease modification and progression slowing
- Ophthalmological applications including corneal wound healing and retinal disorders
- Aesthetic medicine applications for cosmetic wound healing and anti-aging


Conclusion
TB-500 represents a promising therapeutic peptide with demonstrated preclinical efficacy in tissue repair, wound healing, and inflammatory modulation through its unique actin regulation mechanism. Its ability to enhance cellular migration, reduce inflammation, and promote tissue regeneration offers potential advantages in regenerative medicine applications. However, the current evidence base remains predominantly preclinical, with limited human clinical data available.
The absence of FDA approval, regulatory restrictions, and potential safety considerations necessitate careful evaluation and medical supervision for any therapeutic application. The theoretical risk of enhanced angiogenesis in malignant conditions requires particular caution in patient selection and ongoing monitoring protocols.
Healthcare providers considering TB-500 therapy should engage in comprehensive risk-benefit discussions with patients, exploring evidence-based alternatives where available. Future large-scale clinical trials will be essential in establishing safety profiles, optimal dosing regimens, and specific clinical indications for TB-500 in human medicine. Until comprehensive clinical evidence is available, its use should remain limited to research settings and experimental protocols under appropriate medical oversight.
TB-500 SCIENTIFIC
DATA SUMMARY
Disclaimer: This information is provided for educational purposes only and does not constitute medical advice. TB-500 is not approved by the FDA for human therapeutic use. Healthcare providers and patients should consult current regulatory guidelines and conduct thorough risk-benefit analyses before considering any peptide therapy.
The content reflects current scientific literature and regulatory status as of 2025.
