Thymalin
Thymalin is a polypeptide complex derived from the thymus gland of young calves, containing short peptides (2-8 amino acids) that regulate gene expression in immune and hematopoietic cells. Originally developed in the 1970s at the USSR Institute of Gerontology, thymalin represents a targeted approach to counteracting age-related immune decline through restoration of thymic function and T-lymphocyte differentiation.
This peptide complex has gained considerable attention in immunology and geriatric medicine due to its demonstrated efficacy in restoring immune homeostasis, enhancing hematopoiesis, reducing infection susceptibility, and extending healthspan. Thymalin exhibits unique properties as a multi-peptide complex that regulates immune cell differentiation and maintains bioactivity across various clinical applications, making it distinctive among immunomodulatory peptides with decades of clinical use in Eastern Europe.

Overview
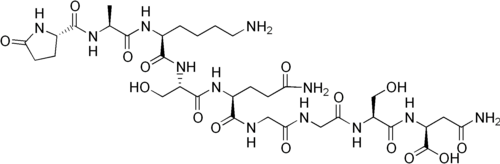
Thymalin contains a heterogeneous mixture of thymic peptides that regulate gene expression in immune and hematopoietic cells. The complex is formulated as a lyophilized injectable solution for parenteral use and demonstrates superior stability and biological activity compared to individual peptide components, with detectability and immune effects persisting for weeks post-administration.

Chemical structure & Properties
- Molecular Formula: Complex mixture of short peptides
- Molecular Weight: 1,000-10,000 Da (heterogeneous mixture)
- Sequence: Short peptides (2-8 amino acids) from calf thymus
- Half-life: Variable based on peptide components
- Stability: Stable at refrigerated conditions (2-8°C) in sealed ampoules
Mechanism of Action
Thymalin exerts its therapeutic effects through multiple interconnected molecular pathways:
Clinical Applications and
Research Evidence

Current Clinical Evidence
Safety Profile and Considerations
Regulatory Status and
Legal Considerations
FDA Status
- Classification: Investigational peptide complex, not approved for therapeutic use
- Approval Status: Not approved for human therapeutic use in the United States
- Development Stage: Approved in Russia, investigational in Western markets
- Regulatory Position: Requires clinical trials for FDA approval pathway
International Status
- Russian Federation: Approved for clinical use as immunomodulatory agent
- Eastern Europe: Widely used in clinical practice
- Western Markets: Available through research channels and compounding pharmacies
Legal Availability
- Commercial Status: Not legally available as prescription medication in the US
- Market Presence: Available through specialized research and compounding sources
- Quality Control: Variable quality and purity verification essential
- Clinical Use: Limited to research settings and experimental protocols in Western countries
Administration and Dosing
Considerations
The Paragon Method: Step-by-Step
Administration Routes
- Intramuscular injection (preferred route)
- Subcutaneous injection (alternative method)
- Site rotation recommended for repeated administration
Clinical Considerations
Important Notes:
- Dosing protocols established through decades of Russian clinical use
- Individual response may vary based on immune status and age
- Medical supervision required for any therapeutic application
- Quality verification essential due to complex peptide mixture

Priority Research Areas
- Expanded clinical trials in Western populations for FDA approval
- Standardized dosing protocols for various clinical applications
- Long-term safety studies in diverse patient populations
- Mechanism elucidation of individual peptide components
- Comparative effectiveness studies against standard immunomodulatory treatments
Emerging Applications
Research is investigating potential applications in:
- Post-viral syndromes including long COVID
- Epigenetic and gene-regulatory effects investigation
- Combination protocols for anti-aging medicine
- Enhanced vaccine response optimization
- Integration with other longevity interventions


Conclusion
Thymalin represents a well-established immunomodulatory peptide complex with demonstrated benefits in clinical studies of immune restoration, hematopoietic support, and healthy aging. Its unique composition as a multi-peptide thymic complex offers advantages in addressing age-related immune decline and treatment-related immunosuppression. The extensive clinical experience in Eastern Europe demonstrates significant therapeutic potential with excellent safety profiles.
The current evidence base includes decades of clinical use with consistent positive outcomes across multiple applications. However, limited Western clinical trials and lack of FDA approval necessitate careful consideration and medical supervision for therapeutic applications. Patients interested in thymalin therapy should engage in thorough discussions with qualified healthcare providers and explore participation in research protocols.
Future research will be critical in establishing thymalin's role in Western medical practice through appropriately designed clinical trials. The combination of established clinical benefits in Eastern Europe, excellent safety profile, and unique immunomodulatory mechanisms positions thymalin as a valuable tool in immunology and geriatric medicine.
Thymalin SCIENTIFIC
DATA SUMMARY
Disclaimer: This information is provided for educational purposes only and does not constitute medical advice. Thymalin is not approved by the FDA for human therapeutic use in the United States. Access may be limited to research protocols and specialized compounding sources. Patients should consult with qualified healthcare providers before considering any peptide therapy.
The content reflects current scientific literature and regulatory status as of 2025.
