Thymosin Alpha-1
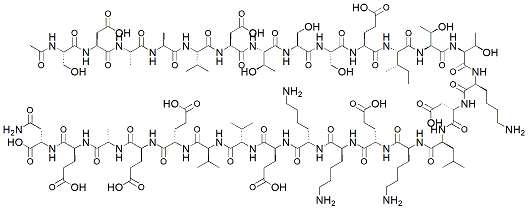
Thymosin Alpha-1 (Tα1) is a synthetic 28-amino acid peptide derived from the naturally occurring thymosin fraction 5 found in the thymus gland, specifically designed to enhance immune system function and regulation. Originally isolated from thymic extracts, Thymosin Alpha-1 represents a critical immunomodulatory peptide that plays a pivotal role in T-cell maturation, natural killer (NK) cell activation, and overall adaptive immune competence. The peptide sequence is: Ac-Ser-Asp-Ala-Ala-Val-Asp-Thr-Ser-Ser-Ile-Lys-Glu-Lys-Phe-Ala-Asp-Asp-Ala-Val-Asp-Thr-Ser-Lys-Thr-Lys-Lys-NH2.
This peptide has gained considerable attention in immunology and infectious disease medicine due to its demonstrated efficacy in enhancing immune responses against viral infections, supporting cancer immunotherapy, and restoring immune function in immunocompromised populations. Thymosin Alpha-1 exhibits excellent bioavailability via subcutaneous administration and maintains therapeutic activity through its ability to modulate both innate and adaptive immunity, making it unique among immunomodulatory peptides.

Overview
Thymosin Alpha-1 demonstrates excellent water solubility and rapid absorption following subcutaneous injection. The peptide exhibits high affinity for immune cell receptors and is metabolized primarily through proteolytic degradation. Despite its relatively short plasma half-life, the peptide's biological effects persist through sustained immune cell activation for extended periods post-administration.

Chemical structure & Properties
- Molecular Formula: C155H247N41O48S
- Molecular Weight: 3108.7 Da
- Sequence: 28 amino acids with N-terminal acetylation
- Half-life: Approximately 2-4 hours (plasma elimination)
- Stability: Stable in aqueous solution but requires careful storage due to proteolytic sensitivity
Mechanism of Action
Thymosin Alpha-1 exerts its therapeutic effects through multiple interconnected immunomodulatory mechanisms:
Clinical Applications and
Research Evidence

Current Clinical Evidence
Safety Profile and Considerations
Regulatory Status and
Legal Considerations
International Regulatory Status
- FDA Status: Investigational status in the United States, available through research protocols
- European Union: Clinical use established in several European countries for specific indications
- Asia-Pacific: Approved for clinical use in multiple countries for viral infections and cancer adjuvant therapy
- Regulatory Position: Varies by jurisdiction with established clinical use in many countries
Clinical Practice Status
- Infectious Disease: Recognized therapy for viral infections in approved jurisdictions
- Oncology: Accepted adjuvant therapy for cancer immunotherapy support
- Geriatric Medicine: Utilized for age-related immune decline management
Legal Availability
- Commercial Status: Available as prescription medication in approved countries
- Research Access: Available through clinical trials and research protocols in other jurisdictions
- Quality Control: Manufacturing standards vary by country and regulatory framework
- Clinical Use: Established clinical practice in multiple international healthcare systems
Administration and Dosing
Considerations
The Paragon Method: Step-by-Step
Clinical Considerations
Important Guidelines:
- Medical supervision essential for appropriate patient selection and monitoring
- Regular immune marker assessment to guide treatment optimization
- Integration with comprehensive infectious disease or oncology care
- Quality verification important for therapeutic efficacy and safety

Priority Research Areas
Clinical Expansion:
- Large-scale randomized controlled trials for additional indications
- Optimal dosing studies for various patient populations and clinical conditions
- Long-term safety evaluation in chronic use applications
- Combination therapy protocols with other immunomodulatory agents
Mechanistic Studies:
- Detailed characterization of immune cell activation pathways
- Investigation of personalized dosing based on individual immune status
- Biomarker development for treatment response prediction and monitoring
- Precision immunotherapy approaches incorporating immune profiling
Emerging Applications
Research is investigating potential applications in:
- Vaccine response enhancement in immunocompromised populations
- Post-surgical immune recovery and infection prevention
- Autoimmune disease management through immune rebalancing
- Longevity medicine and healthy aging through immune system support
- Combination immunotherapy protocols for complex immune conditions


Conclusion
Thymosin Alpha-1 represents a well-established immunomodulatory peptide with demonstrated clinical efficacy in viral infections, cancer adjuvant therapy, and age-related immune decline. Its mechanism of action through T-cell and NK cell activation, cytokine modulation, and immune system restoration provides significant therapeutic benefits with an excellent safety profile established through decades of clinical use.
The peptide's international regulatory approval and established clinical practice in multiple healthcare systems provide healthcare providers with confidence in its therapeutic applications. The extensive clinical evidence supporting its use in viral infections, oncology, and geriatric medicine positions it as a valuable therapeutic option for immune system enhancement.
Healthcare providers should approach Thymosin Alpha-1 therapy with comprehensive immune system assessment and appropriate monitoring protocols. The peptide's established safety record and proven clinical efficacy make it suitable for diverse patient populations requiring immune system support and enhancement.
Future research will likely expand approved indications and optimize treatment protocols, but current evidence establishes Thymosin Alpha-1 as a proven immunomodulatory therapy with significant clinical value under appropriate medical supervision.
THYMOSIN ALPHA-1 SCIENTIFIC
DATA SUMMARY
Disclaimer: This information is provided for educational purposes only and does not constitute medical advice. Thymosin Alpha-1 regulatory status varies by jurisdiction. Patients should consult with qualified healthcare providers familiar with local regulations before considering any immunomodulatory therapy.
The content reflects current scientific literature and international clinical experience as of 2025.
