Tirzepatide
Tirzepatide is a novel synthetic peptide representing a groundbreaking advancement in metabolic medicine through its dual agonist activity at glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptors. Originally developed as a dual incretin receptor agonist, tirzepatide represents a paradigm shift in obesity and diabetes management by addressing both glycemic control and weight management through complementary mechanisms that offer superior efficacy compared to single-receptor targeting approaches.
This peptide has gained considerable attention in endocrinology and obesity medicine due to its demonstrated efficacy in achieving substantial weight loss (15-22% of body weight) and exceptional glycemic control in patients with type 2 diabetes and obesity. Tirzepatide exhibits unique properties through its dual-incretinmimetic mechanism and maintains bioactivity across various metabolic pathways, making it distinctive among therapeutic peptides with FDA approval for both diabetes and weight management applications.

Overview
Tirzepatide incorporates structural modifications including a fatty acid side chain that enables albumin binding, significantly extending the peptide's half-life compared to native incretin hormones. The peptide is metabolized through proteolytic degradation and renal elimination, with detectability and therapeutic effects persisting for up to one week post-administration using standard monitoring methods.

Chemical structure & Properties
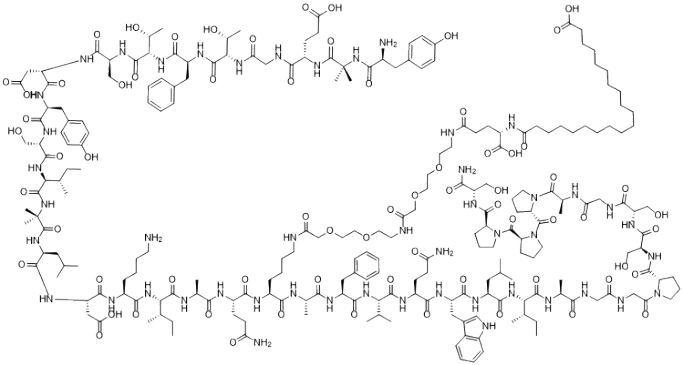
- Molecular Formula: C225H348N48O68
- Molecular Weight: 4,813 Da
- Sequence: 39-amino acid peptide with fatty acid side chain
- Half-life: Approximately 5 days (enabling once-weekly dosing)
- Stability: Stable peptide formulation requiring refrigeration
Mechanism of Action
Tirzepatide exerts its therapeutic effects through multiple interconnected molecular pathways:
Clinical Applications and
Research Evidence

Current Clinical Evidence
Safety Profile and Considerations
Regulatory Status and
Legal Considerations
FDA Status
- Classification: FDA-approved prescription medication
- Approval Status: Approved for type 2 diabetes (Mounjaro®) and chronic weight management (Zepbound®)
- Approval Dates: Diabetes approval May 2022, weight management approval November 2023
- Regulatory Position: Full FDA approval with established safety and efficacy
International Status
- EMA: Approved for diabetes management in European Union
- Health Canada: Approved for diabetes management
- Global Markets: Multiple international regulatory approvals ongoing
Legal Availability
- Commercial Status: Legally available as prescription medication
- Market Presence: Available through licensed healthcare providers and pharmacies
- Quality Control: Manufactured under strict pharmaceutical quality standards
- Clinical Use: Standard medical practice for approved indications
Administration and Dosing
Considerations
The Paragon Method: Step-by-Step
Administration Routes
- Subcutaneous injection via prefilled pen device (only approved route)
- Same day each week administration
- Injection site rotation (thigh, abdomen, upper arm)
- Oral Administration: Not recommended due to poor bioavailability
Clinical Considerations
Important Notes:
- FDA-approved dosing guidelines must be followed for therapeutic use
- Individual response monitoring required for dose optimization
- Medical supervision required for all therapeutic applications
- Proper injection training and technique essential for safety and efficacy

Priority Research Areas
- Long-term cardiovascular outcomes studies (SURPASS-CVOT ongoing)
- Optimal dosing strategies for different patient populations
- Long-term safety assessment with extended follow-up periods
- Comparative effectiveness research against other obesity and diabetes treatments
- Investigation of additional metabolic and therapeutic applications
Emerging Applications
Research is investigating potential applications in:
- Non-alcoholic fatty liver disease treatment
- Cardiovascular disease prevention in high-risk populations
- Metabolic syndrome management beyond diabetes and obesity
- Combination therapies for enhanced metabolic outcomes
- Pediatric obesity and diabetes applications


Conclusion
Tirzepatide represents a revolutionary therapeutic peptide with demonstrated benefits in clinical studies of type 2 diabetes and obesity management. Its unique dual incretin receptor mechanism offers unprecedented advantages in metabolic medicine with superior efficacy compared to existing treatments. The comprehensive clinical trial program and FDA approval establish tirzepatide as a first-line therapeutic option for appropriate patients.
The current evidence base includes robust Phase III clinical trial data with over 17,000 patients demonstrating consistent safety and efficacy across multiple studies. However, continued post-marketing surveillance and ongoing research will further establish optimal treatment strategies and long-term outcomes. Patients interested in tirzepatide therapy should engage in thorough discussions with qualified healthcare providers to assess appropriateness and establish treatment plans.
Future research will continue to expand understanding of tirzepatide's therapeutic potential and optimal clinical applications. The combination of proven clinical benefits, established
Tirzepatide SCIENTIFIC
DATA SUMMARY
Disclaimer: This information is provided for educational purposes only and does not constitute medical advice. Tirzepatide is FDA-approved for type 2 diabetes and chronic weight management under the brand names Mounjaro® and Zepbound®. Patients should consult with qualified healthcare providers before considering any peptide therapy.
The content reflects current scientific literature and regulatory status as of 2025.
